Herpes Vaccine Research in 2025
Herpes simplex virus (HSV) infections are widespread globally, with an estimated 3.7 billion people infected with HSV-1 and 500 million with HSV-2 . HSV-1 is mainly associated with oral herpes (cold sores), while HSV-2 typically causes genital herpes. Despite decades of research, no licensed vaccines are available to prevent or treat these infections. This article provides an in-depth review of herpes vaccine research in 2025, covering clinical trials, vaccine types, preclinical studies, development progress, challenges, funding, and expert opinions on the future of this field.
2/17/20255 min read
Clinical Trial Results in 2025
As of early 2025, several herpes vaccine candidates are undergoing clinical trials, but none have been authorized by major regulatory agencies worldwide . Promising candidates include:
Moderna's mRNA-1608: This mRNA vaccine candidate targets HSV-2 and is currently in a Phase 1/2 clinical trial. The study, which began in September 2023, is evaluating the safety and immune response of different doses in adults with a history of recurrent genital herpes . Results are expected in June 2025 .
BioNTech's BNT163: This mRNA-based vaccine candidate encodes three HSV-2 glycoproteins to prevent viral entry and spread. A Phase 1 clinical trial launched in December 2022 is studying the vaccine in adults without a history of symptomatic genital herpes . This trial is expected to be completed in December 2025 .
Rational Vaccines' RVx-201: This live-attenuated HSV-2 vaccine candidate is designed to reduce the frequency and severity of outbreaks. An observational clinical study (RVx-001-PSS) is being conducted in England . In animal studies, RVx-201 significantly reduced symptomatic days and recurrent genital herpes lesions .
Assembly Biosciences' ABI-5366: This candidate is an advanced helicase-primase inhibitor targeting healthy participants and those with recurrent genital herpes. A Phase 1a/1b clinical trial found ABI-5366 to be well-tolerated with a pharmacological profile supporting potential once-weekly or even once-monthly dosages .
The use of mRNA technology in two of these leading candidates (mRNA-1608 and BNT163) highlights the growing importance of this approach in vaccine development. mRNA vaccines offer potential advantages in terms of rapid development, scalability, and the ability to induce both antibody and T-cell responses .
While these candidates show potential, setbacks have occurred. GSK plc recently announced that its therapeutic HSV vaccine candidate, GSK3943104, did not meet the primary efficacy objective in a Phase I/II trial . Despite this, no safety concerns were observed, and GSK plans to continue safety monitoring .
Challenges and Setbacks in Herpes Vaccine Research
Developing a herpes vaccine faces significant challenges:
Viral Latency: Herpesviruses can establish latent infections, remaining undetected by the immune system for extended periods . This latency makes it difficult for vaccines to effectively target and eliminate the virus.
Limited Immune Response: The human immune system does not mount a strong response to herpes infections, hindering the development of effective vaccines .
Difficulty Translating Animal Studies to Humans: While some vaccine candidates have shown promise in animal studies, they have not been as successful in human trials . This highlights the need for further research to understand the complexities of human immune responses to herpes.
Need for Broad Immune Response: Vaccine development should focus on strategies that elicit a broad immune response recognizing a diverse set of viral antigens . This is crucial because herpesviruses have evolved mechanisms to evade the immune system, and a narrowly focused response may not be sufficient for effective protection.
Economic Burden: Genital herpes infections result in significant economic losses, estimated at $35 billion annually . This includes costs associated with treatment, lost wages, and reduced quality of life. Developing a vaccine could significantly alleviate this economic burden.
Interplay with HIV: The prevalence of HSV poses challenges related to superinfections, particularly with HIV. An effective HSV vaccine could potentially reduce HIV incidence by 30-40% over 20 years . This underscores the importance of herpes vaccine research in the broader context of public health.
These challenges contribute to the ongoing struggle to develop a successful herpes vaccine.
Progress of Herpes Vaccine Development in 2025
Despite the challenges, progress is being made in herpes vaccine development. The WHO published its preferred product characteristics for alpha-herpesvirus vaccines in 2024, providing a framework for vaccine development . Several vaccine candidates are in clinical trials, with some showing promising results in preclinical studies . For example, Moderna's mRNA-1608 demonstrated high efficacy in animal models, reducing genital disease by 85-100% .
The WHO is also actively involved in raising awareness about the costs of genital herpes infections and promoting research and development of vaccines . This includes highlighting the economic burden of the disease and advocating for increased investment in prevention tools.
Preclinical Studies
Preclinical studies play a crucial role in herpes vaccine development by providing valuable insights into the virus's behavior and potential vaccine targets. One area of preclinical research involves the use of AlphaFold, a protein structure prediction tool, which includes two additional Herpesviruses (PrV and MHV68) . This technology can help researchers understand the structure of viral proteins and design vaccines that effectively target them.
Another promising avenue in preclinical research is the exploration of combination therapy with monoclonal antibodies. A study published in May 2024 highlighted the potential of combining two monoclonal anti-gB IgGs (HDIT101 and HDIT102) for treating HSV-1 and HSV-2 infections . This approach could lead to more effective treatments and potentially inform the development of new vaccine strategies.
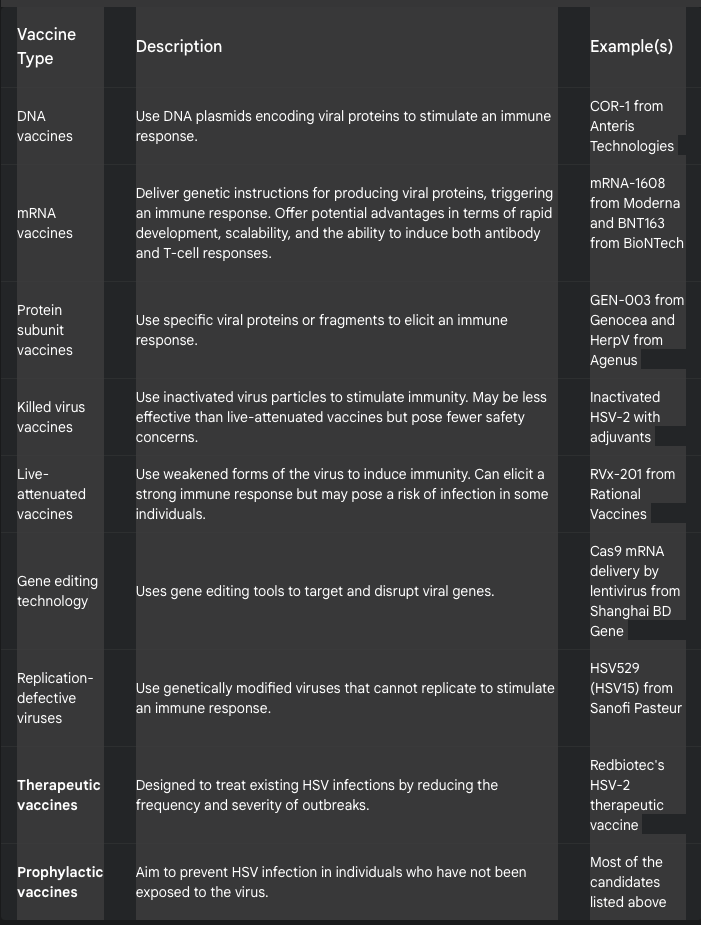
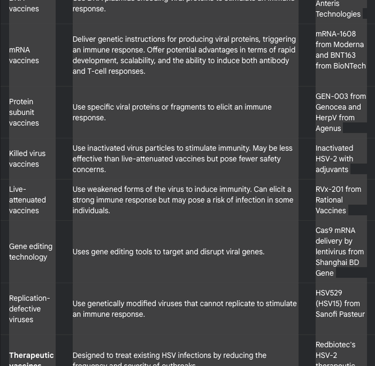
Types of Herpes Vaccines Being Researched
Herpes vaccine research in 2025 employs various approaches, each with its own potential advantages and disadvantages:
This diverse range of approaches reflects the ongoing efforts to find an effective herpes vaccine.
Funding and Support for Herpes Vaccine Research
Funding and support for herpes vaccine research come from various sources:
Government Agencies: The National Institute of Allergy and Infectious Diseases (NIAID) offers funding opportunities for research on new therapeutic strategies for genital herpes, including vaccines . This highlights the government's commitment to supporting research in this critical area.
Non-profit Organizations: Organizations like the WHO are working to advance research and development of herpes vaccines . Their efforts include raising awareness, providing guidance for vaccine development, and advocating for increased investment in the field.
Private Companies: Pharmaceutical companies like Moderna, BioNTech, and Rational Vaccines are investing in herpes vaccine research and development . These companies play a crucial role in translating research findings into clinical trials and ultimately bringing vaccines to market.
Continued funding and support from these diverse sources are crucial for overcoming the challenges and advancing the field.
Expert Opinions and Analysis on the Future of Herpes Vaccine Development
Experts emphasize the need for continued research to understand the complex immune responses to herpes and develop effective vaccines . Key areas of focus include:
Broad Immune Response: Vaccines should elicit a broad immune response that targets multiple viral antigens . This is crucial to overcome the virus's ability to evade the immune system.
T-cell Responses: Virus-specific T-cell responses are crucial for an effective therapeutic vaccine . T-cells can directly kill infected cells and provide long-lasting immunity.
Mucosal Immunity: Strong immune responses at the site of infection, such as the genital tract, are essential . This can help prevent viral entry and reduce transmission.
Combination Approaches: Combining different vaccine strategies, such as prime/pull approaches with immune checkpoint blockade, may enhance efficacy . This could involve priming the immune system with a vaccine that induces a systemic response and then boosting it with a mucosal vaccine that targets the site of infection. Immune checkpoint blockade could further enhance the response by preventing the virus from suppressing the immune system.
Addressing Both HSV-1 and HSV-2: An effective genital herpes vaccine needs to protect against both HSV-1 and HSV-2 . This is because both viruses can cause genital herpes, and cross-protection is essential for comprehensive prevention.
Experts also highlight the potential of novel approaches, such as nanotechnology and genomic editing, in herpes vaccine development . Nanotechnology could be used to deliver vaccines more effectively to target cells, while genomic editing could be used to disrupt viral genes and prevent infection.
Synthesis and Conclusion
Herpes vaccine research in 2025 is characterized by ongoing clinical trials, diverse vaccine approaches, and a growing understanding of the challenges involved. While no licensed vaccines are currently available, promising candidates are emerging, particularly those utilizing mRNA technology. These candidates offer the potential for rapid development, scalability, and the ability to induce both antibody and T-cell responses.
However, significant challenges remain, including viral latency, limited immune response, and the difficulty of translating animal studies to humans. To overcome these hurdles, researchers are focusing on strategies to elicit a broad immune response, enhance T-cell responses, and target mucosal immunity. Combination approaches and novel technologies, such as nanotechnology and genomic editing, also hold promise for the future of herpes vaccine development.
The future outlook for herpes vaccine development is cautiously optimistic. While a "cure" may still be years away, the current research landscape suggests that an effective vaccine to prevent or significantly reduce the severity of herpes infections is a realistic goal. Continued research, funding, and collaboration are essential to realize this goal and improve the lives of millions affected by herpes.